IsoMDx COVID-19 Kit(30minutes inspection completed by PT-PCR
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- BZ IsoMDx COVID-19 Kit
- Payment Terms
- Others
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- pcr, covid, real time reverse transcription, loop-mediated
- Category
- Medical Analyzer , Medical Test Kit

People Medical
- Verified Certificate
-
4

| Product name | IsoMDx COVID-19 Kit(30minutes inspection completed by PT-PCR | Certification | CE |
|---|---|---|---|
| Category |
Medical Analyzer
Medical Test Kit |
Ingredients | - |
| Keyword | pcr , covid , real time reverse transcription , loop-mediated | Unit Size | 23.0 * 9.0 * 13.0 cm |
| Brand name | BZ IsoMDx COVID-19 Kit | Unit Weigh | 400 g |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | 9031 |
Product Information
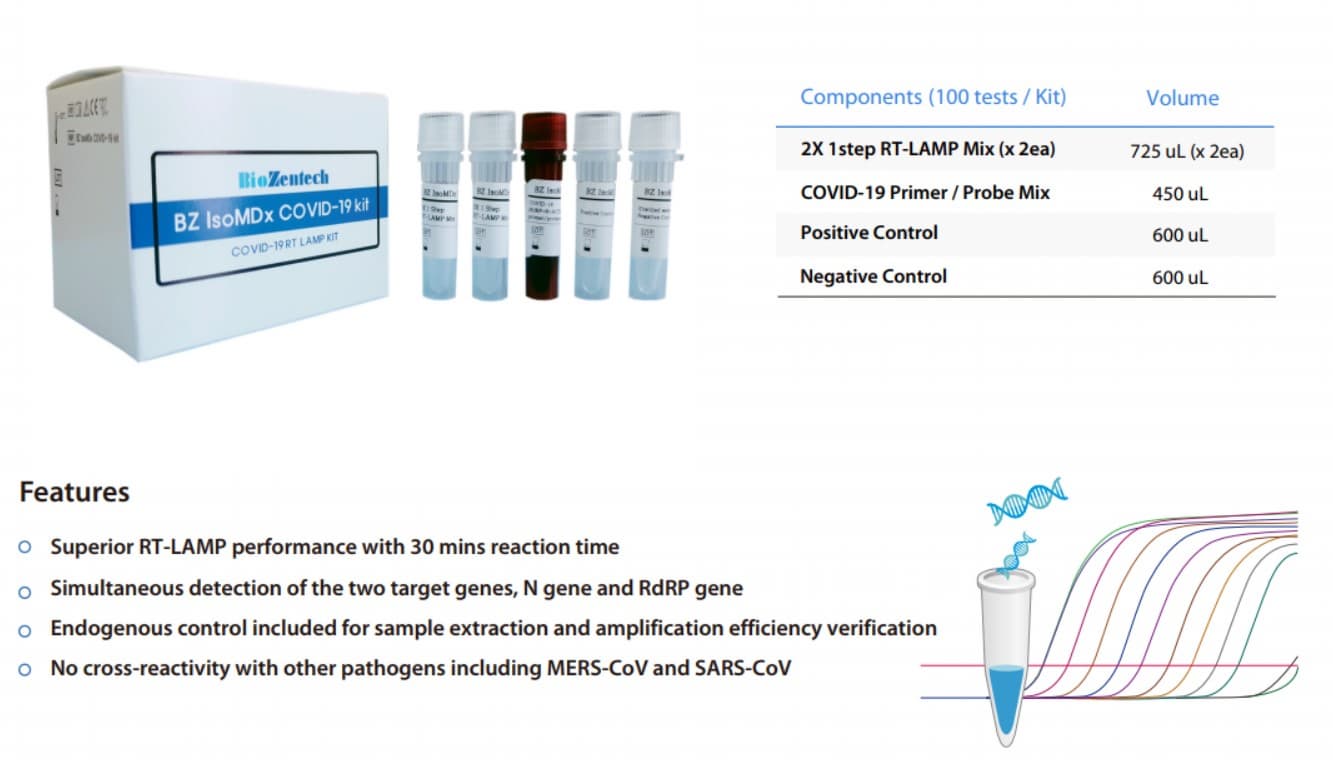
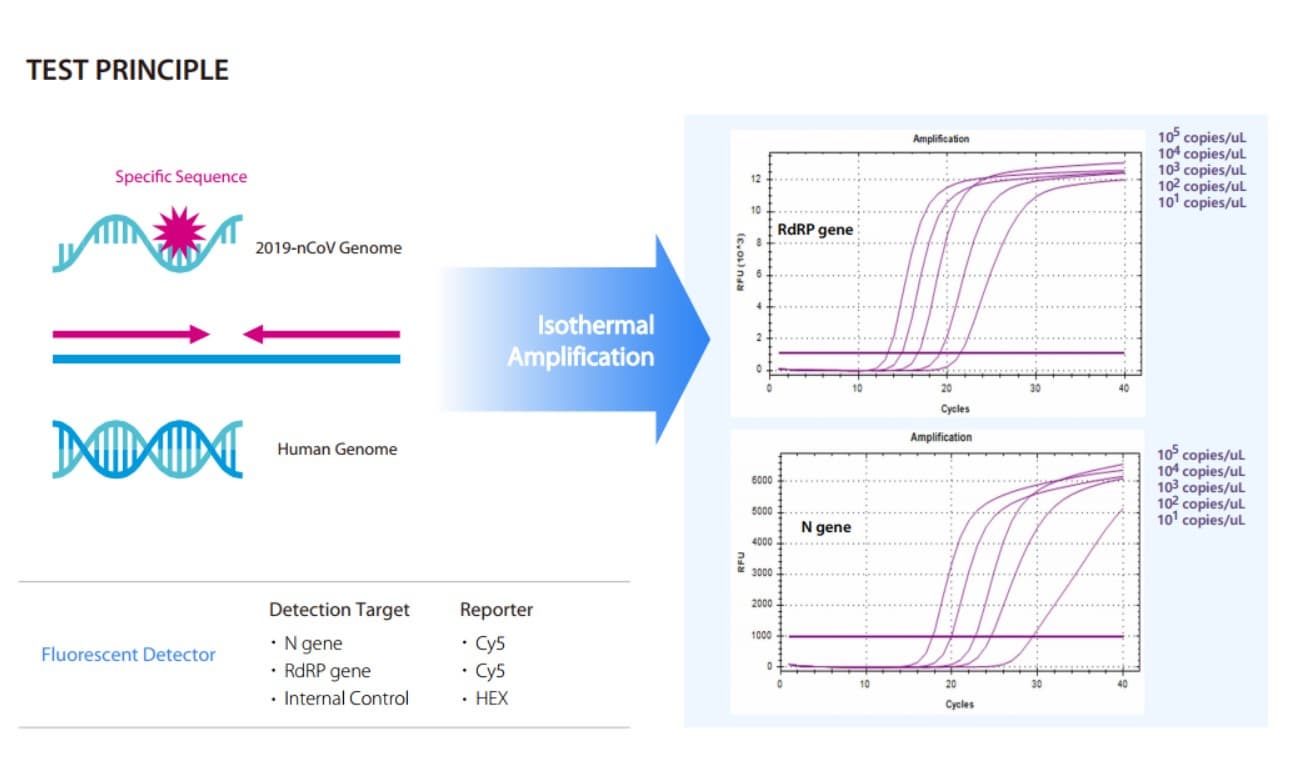
BZ IsoMDx COVID-19 kit is an in vitro diagnostic using RT-LAMP(Real time Reverse Transcription Loop-mediated
Isothermal Amplification) assay for qualitative detection of coronavirus disease (COVID-19) from RNA extracted
from human nasopharyngeal swab, oropharyngeal swab and sputum
Detect two gene regions and simultaneously detect N gene as well as RdRP gene of SARS-CoV-2.
Endogenous control included for sample extraction and amplification efficiency verification
CONCLUSION
BZ IsoMDx COVID-19 kitshowed tsreults inlowest conetraion as1/04 diluted RNAin twospecimns. • In twospecimns, AlpexTM 2019-nCoV Asay howed tsreults inlow cnetraion as1/04 diluted RNA(asopharyngeal
swab), 1/05 diluted RNA(Sputm). • BZ IsoMDx COVID-19 kitdectd heSAR-CoV2 equaly toAlpexTM 2019-nCoV Asay
BZ IsoMDx COVID-19 kits enable todetc SAR-CoV2 asmuch asthe AlpexTM 2019-nCoV Asay
Analytical sensitivity (Limit of Detection) To determine the analytical sensitivity of BZ IsoMDx COVID-19 kit, the upper respiratory tract specimens (Nasopharyngeal swab) were diluted with internal standard material, and was tested 20 times.
The concentration of 100% or more positive result was determined as the minimum detection limit.
The limit of detection is 102 copies/µL for the specimens from the upper respiratory tract regardless of the PCR systems including CFX96TM Dx System (Bio-Rad)..
Analytical sensitivity (Cut off Value) The cut off value was determined as 30 based on the Ct value, which was set using the LOD (Limit of detection) test result value.
Analytical specificity (Cross Reactivity) To evaluate the cross reactivity of BZ IsoMDx COVID-19 kit, the possible cross reactive pathogens as listed in the table below were tested 3 repeated times.
As a result, no cross reactivity was observed for the pathogens showing the similar symptoms or alpha coronavirus.
- Mycoplasma Pneumoniae - Streptococcus Pneumoniae - Influenza A Virus H1 - Influenza A Virus H3 - Influenza A Virus H1N1 - Influenza B Virus1 - Human Respiratory Syncytial A Virus (RSV) - Human Respiratory Syncytial B Virus (RSV) - Adeno Virus - Para Influenza Virus - Human Corona Virus, OC43 - Human Corona Virus, NL63 - Human Corona Virus, 229E - Human Boca Virus - Meta Pneumoniae - Huma Rhino Virus - Human Entero Virus - Staphylococcus Aureus Strain - Middle East Respiratory Syndrome Corona Virus - Human Astro Virus
http://www.peoplemedical.co.kr
Analytical specificity (Interference)
To test the effect of the possible interfering substances BZ IsoMDx COVID-19 kit was tested 3 repeated times using specimen prepared by adding the materials listed below.
(Mucin 1%, Acetyl salicylic Acid 15mg/mL, NaCl 7.4mg/mL, Oxymetazoline 20%, Hemoglobin 0.2%, Whole blood 3%) Precision (Reproducibility)
To evaluate reproducibility of BZ IsoMDx COVID-19 kit for nasopharyngeal swab from the upper respiratory tract from the lower respiratory tract, one run of test were performed each day.
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Others | Shipping time | Negotiable |

- President
- Kim Sune
- Address
- 1166,104dong 701 room, Pyeongtaek-si, Gyeonggi-do, Korea
- Product Category
- Medical Test Kit,Other Health Care Products
- Year Established
- 2019
- No. of Total Employees
- 1-50
- Company introduction
-
We, People Medical, will do our best to block infectious diseases with better products and materials for K-POE.Our product is a 100% domestic disposable mask made with Korean manufacturing technology by securing a large amount of domestic fabric.
It passed the tests of the Korea Apparel Testing and Research Institute (KATRI) and the Korea Textile Material Research Institute (KOTERI).
Korea-Prevention Of Epidemics has been filed for a patent.Accordingly, we would like to inform the world of our publicity.
We are pleased to introduce BZ COVID-19 Ag Rapid Test We want to export To many countries
We have received CE certification for the antigen (Ag) test kit among the corona 19 diagnostic test methods in Korea, and we have contacted you to export to Europe, Asia etc
This Corona 19 test kit test (Ag) detects a pharyngeal sample after 3 days, and it is known that the clinical sensitivity is 97% and the specificity is 99.3%.
The manufacturer of this diagnostic kit is http://www.biozentech.co.kr, and the representative of the manufacturer is Professor Chae-Seung Lim, Department of Laboratory Medicine, Korea University Hospital.
- Main Markets
-
 Austria
Austria
 France
France
 Hungary
Hungary
 Spain
Spain
 Sweden
Sweden
 Turkey
Turkey
 U. Kingdom
U. Kingdom
- Main Product


































 South Korea
South Korea
Medium_for_collecting_corona_2.jpg)

_2.jpg)